I have decided to dedicate this post to the specific analgesic potential of keratinocytes because it is something that is hardly talked about in the world of wound healing and yet it is observed daily in clinical practice by all of us who graft wounds:)
In several blog posts I talk about the pain reduction we achieve when grafting painful wounds. In fact, one of them is directly entitled “Punch grafting as a painkiller for painful ulcers” in which I discuss the results of a prospective study in which we included 136 patients with wounds of different aetiologies, in whom we measured the intensity of pain, with a visual analogue scale (VAS), before punch grafting (t0) and in the following 3 weeks visits (t1,t2,t3). The primary endpoint was pain reduction. We found that pain reduction was clinically and statistically significant throughout the time of registration and, strikingly, we found no correlation between the percentage of graft attachment and pain reduction.
A sentence I repeat in these posts (also in classes or lectures I give) is: “Even if not all punch grafts do not adhere to the wound bed, they release growth factors and cells that promote healing and reduce pain”.
And why don’t I give more details about the exact mechanism of pain reduction? Because they are not known… In fact, I have not found any articles that analyse it… However, I have found evidence on the general role that keratinocytes may play in pain modulation. Although studies are not specific to grafts and in the reduction of pain with punch grafts there are many more factors potentially involved (fibroblasts, protein matrix, other proteins and growth factors), I think it is important to talk about the specific role of the keratinocyte, since using epidermal grafts (See post: “Differences between epidermal and dermo-epidermal punch grafts”) or keratinocyte cultures there is also evidence of pain reduction.
Available evidence suggests that keratinocytes can communicate with the nervous system via the opioid receptor system. Indeed, studies have shown that cells such as keratinocytes and fibroblasts play an important role in endogenous peripheral antinociception, as they are able to synthesise and secrete peptides such as enkephalins, a type of opioid neurotransmitter. The release of proinflammatory cytokines, especially IL-1b, induces the expression and release of opioids by these cells, which, once bound to receptors on peripheral nerve fibres, inhibit the release of pain neurotransmitters such as substance P.1,2.
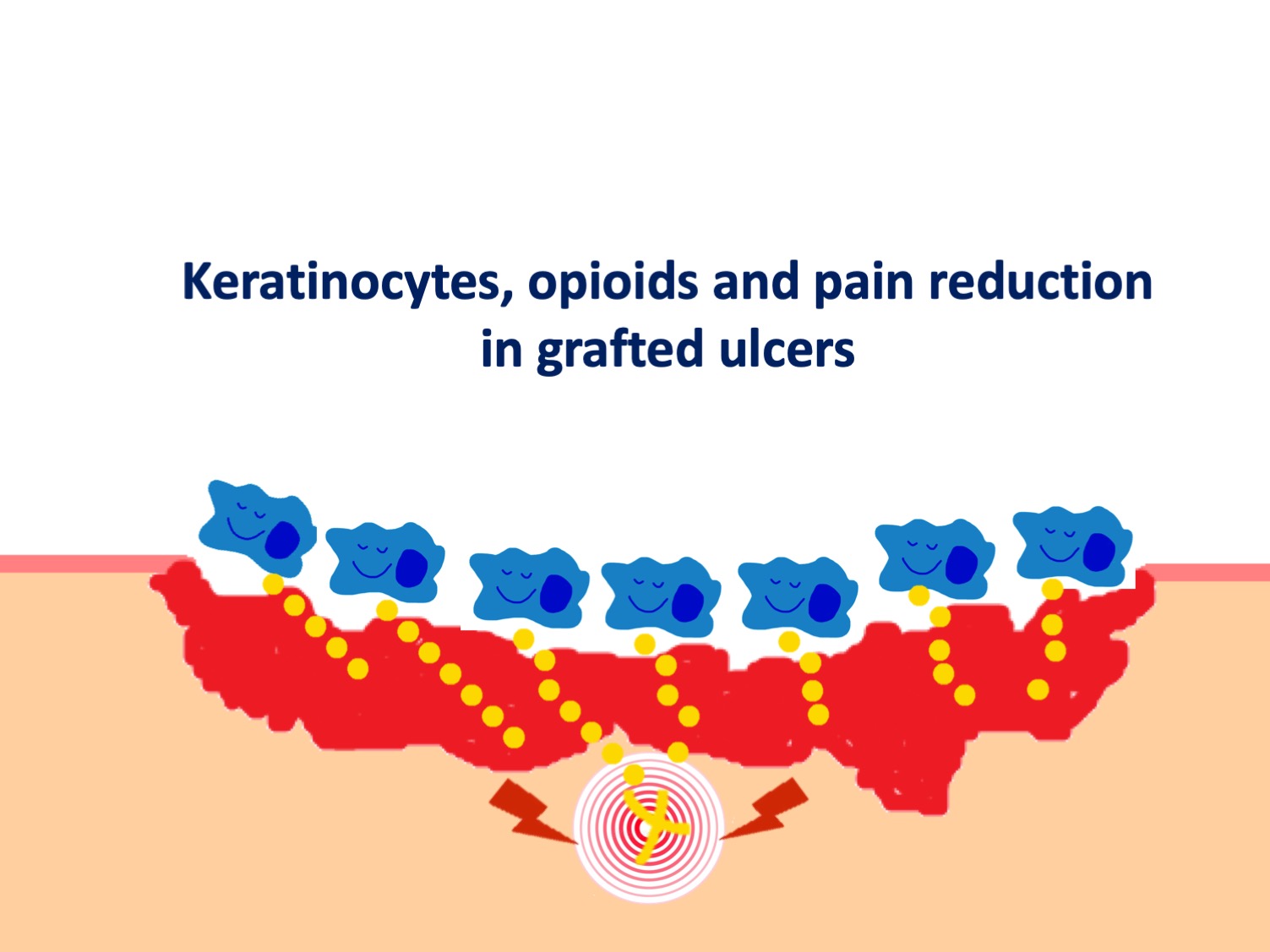
A recent study has shown that keratinocyte and fibroblast culture supernatant promotes analgesia during inflammatory pain induced in a rat model of hyperalgesia, corroborating previous studies. In this study, administration of a non-selective opioid antagonist (naloxone) inhibited the antihyperalgesic effect of keratinocyte culture supernatant, demonstrating that the effect of keratinocyte culture supernatant is mediated by the release of opioids.3
Thus, the release of endogenous opioids by keratinocytes, stimulated by the pro-inflammatory wound environment, may be one of the pathways by which punch grafts have such striking analgesic power.
An exciting topic for further research!
Also available in: Español (Spanish)