The idea of talking about iron and venous ulcer came to me when I read an article that has just been published on the potential relationship between iron deficiency and venous leg ulcer, suggesting the possible role of iron loss from wound exudate: “Does localized iron loss in venous disease lead to systemic iron deficiency? A descriptive pilot study “1. In addition, the authors of this paper have also just published a review on iron deposits, anaemia and venous ulcers: “An overview of the relationship between anaemia, iron, and venous leg ulcers “2
Since there is still much to be researched on the iron-deficit/venous ulcer relationship and the actual impact of anemia on healing, let’s talk about a topic that has been studied in more detail, although the available evidence is not very robust either: The role of local iron deposits in the evolution of chronic venous insufficiency.
Which functions has iron in the body?
Iron is the most abundant trace element in our organism and plays a fundamental role in many cellular functions. It is an essential part of haemoglobin as it facilitates the transport of oxygen to tissues. It participates in multiple metabolic processes, since it is part of enzymes and other molecular complexes. In addition, it also has an important function in tissues with a lot of cell turnover, such as skin, since it regulates DNA replication.
Despite being a fundamental metal for life, in high quantities it can be toxic. This is because free iron has the ability to yield or donate electrons easily (cyclic shift from the Fe2+ to Fe3+ state) and can catalyze free radical reactions and increase oxidative stress.
Our body is responsible for limiting this potential damage by storing iron in protein complexes, such as haemoglobin, transferrin or ferritin. In addition, there are many regulatory mechanisms to maintain an adequate balance between free (hazardous) and protein bound iron. However, these regulatory mechanisms are not sufficient when there is uncontrolled iron overload, both at systemic and local levels.
Why do cutaneous iron deposits occur on the legs?
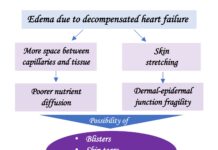
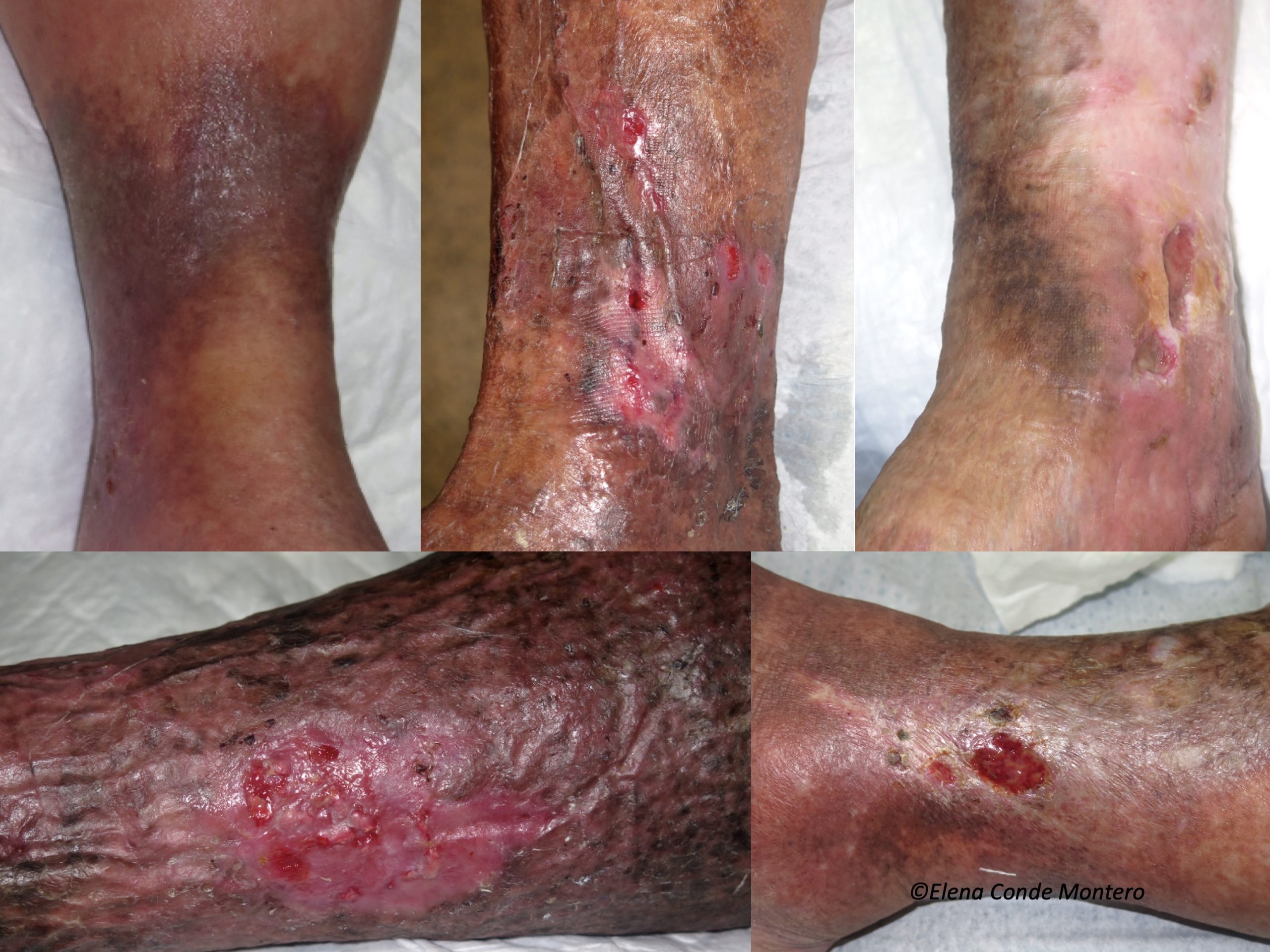
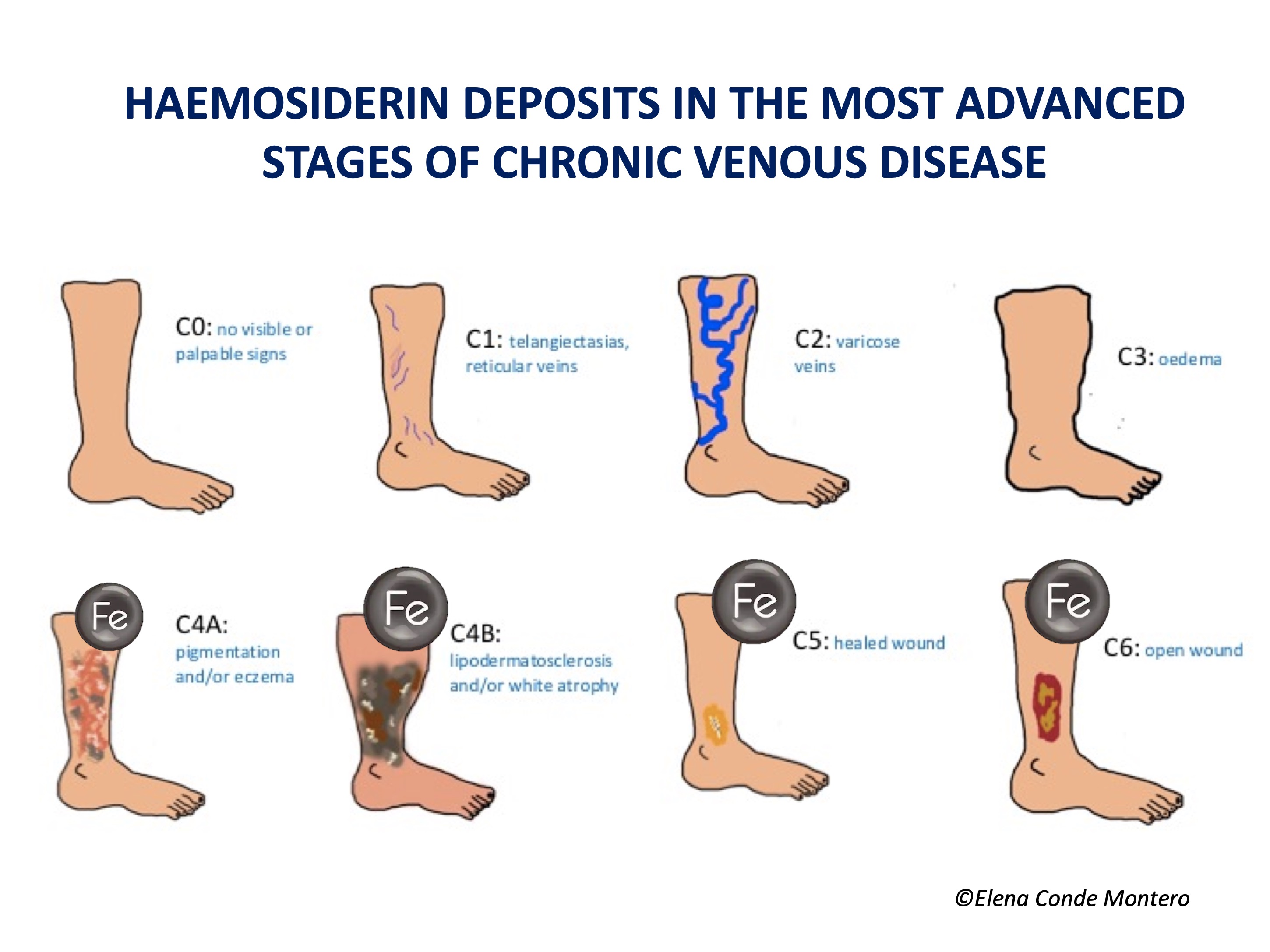
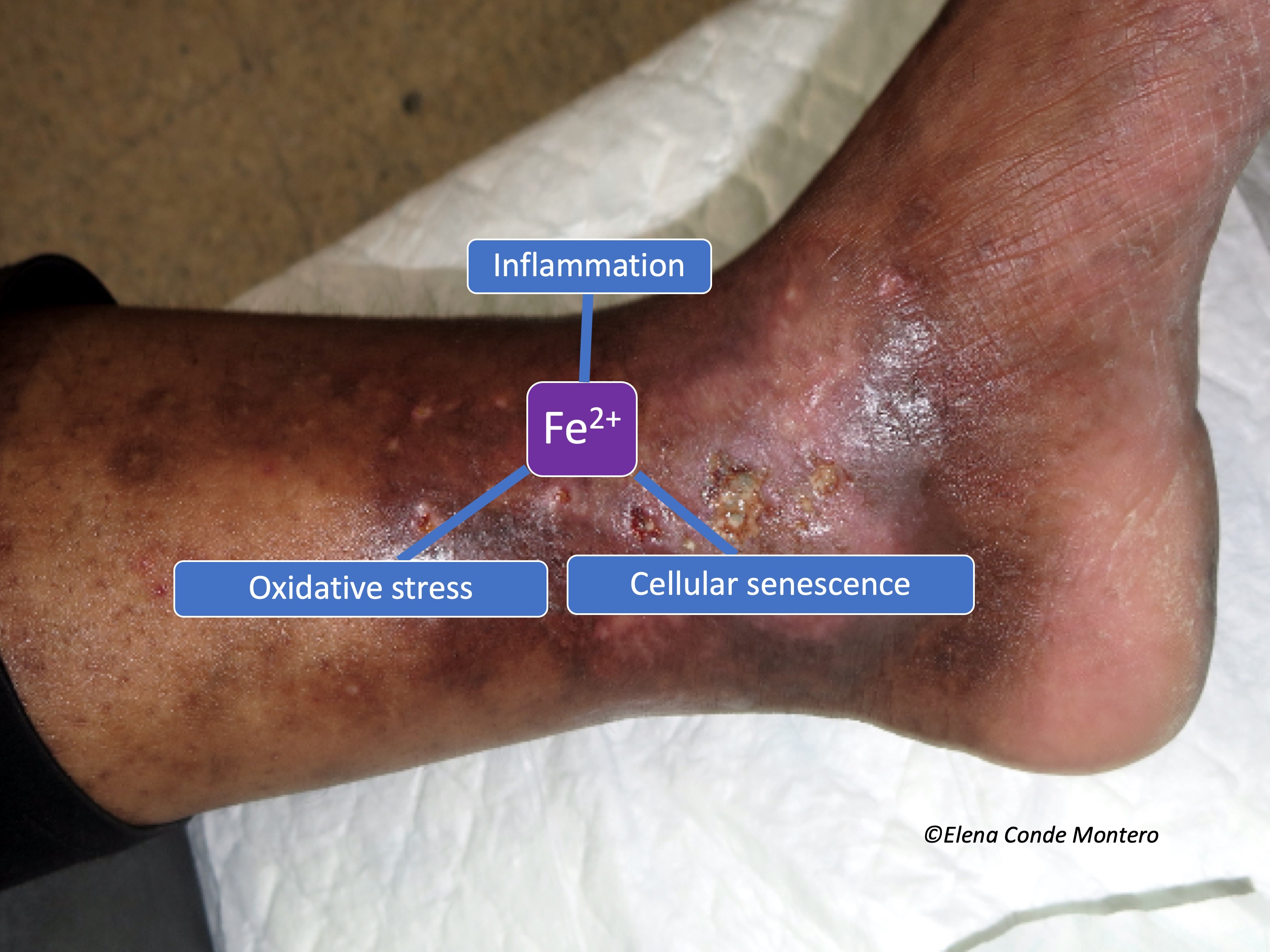
Uncontrolled venous hypertension, which causes chronic venous insufficiency (CVI), leads to extravasation of red blood cells into the skin. These extravasated red blood cells are ingested and degraded by macrophages in the dermis, which release and store iron inside them, first in the form of ferritin and then, when saturated, in the protein complex called hemosiderin. These iron deposits, in addition to melanin deposits (melanocytes are activated by inflammatory processes affecting the skin, such as CVI), produce a brownish coloration in the skin, which is called ochre dermatitis and is one of the clinical characteristics of stage C4 of the CEAP classification of chronic venous disease (See post “CEAP classification of chronic venous disorders: let’s all speak the same language“). What is the weight, separately, of haemosiderin and melanin in the ochre colour of these legs? This is a difficult question to answer, since hemosiderin activates melanin production by melanocytes…
The problem of this hyperpigmentation is not only aesthetic. Iron deposits in the skin seem to have great importance in the evolution of the venous disease and, therefore, of the venous ulcer. In fact, in our clinical practice we observe that leg ulcers with extensive deposits of hemosiderin are more resistant to treatment.
What has been researched on hemosiderin deposits and the evolution of venous disease?
Published studies are scarce and include few patients. Caggiati’s group performed 2 studies in which they analysed haemosiderin deposits in skin biopsies of legs in different stages of chronic venous disease.3,4 In both studies, most patients with normal skin colour did not have haemosiderin deposits in the biopsy (detected by Perls staining). In patients with hyperpigmentation (C4), haemosiderin deposits were only found in those with higher pigmentation. In fact, in the initial stages, these authors associate brownish colour with a greater amount of melanin, since, as we have said, melanocytes can be activated in all cutaneous inflammatory processes. In more advanced stages, such as lipodermatosclerosis (C4b) and venous ulcer (C6) these deposits were always found. Patients with healed ulcers (C5) were not included, so no conclusions can be drawn from this group. The authors conclude that, despite not being able to draw physiopathological conclusions, it is clear that the progressive skin changes of chronic venous disease are associated with the presence of haemosiderin deposits. They also suggest that there may be some genetic condition that justifies a greater deposit of iron (and also melanin) in certain patients.
Regarding to the few studies comparing iron levels in chronic vs. acute wound exudate and in the edges vs. perilesional tissue, the variability between the methods used for detection stands out. However, the results point to a higher amount of iron in wound bed and edge of chronic ulcers.
What role may iron deposits play in the chronicity of wounds?
The differentiation of macrophages and their passage from M1 (pro-inflammatory) to M2 (anti-inflammatory) type is fundamental in the transition from the inflammatory to the proliferative phase of the healing process. Iron seems to be involved in these changes since, when ingested by M1, it maintains its activity and hinders its transformation into M2.
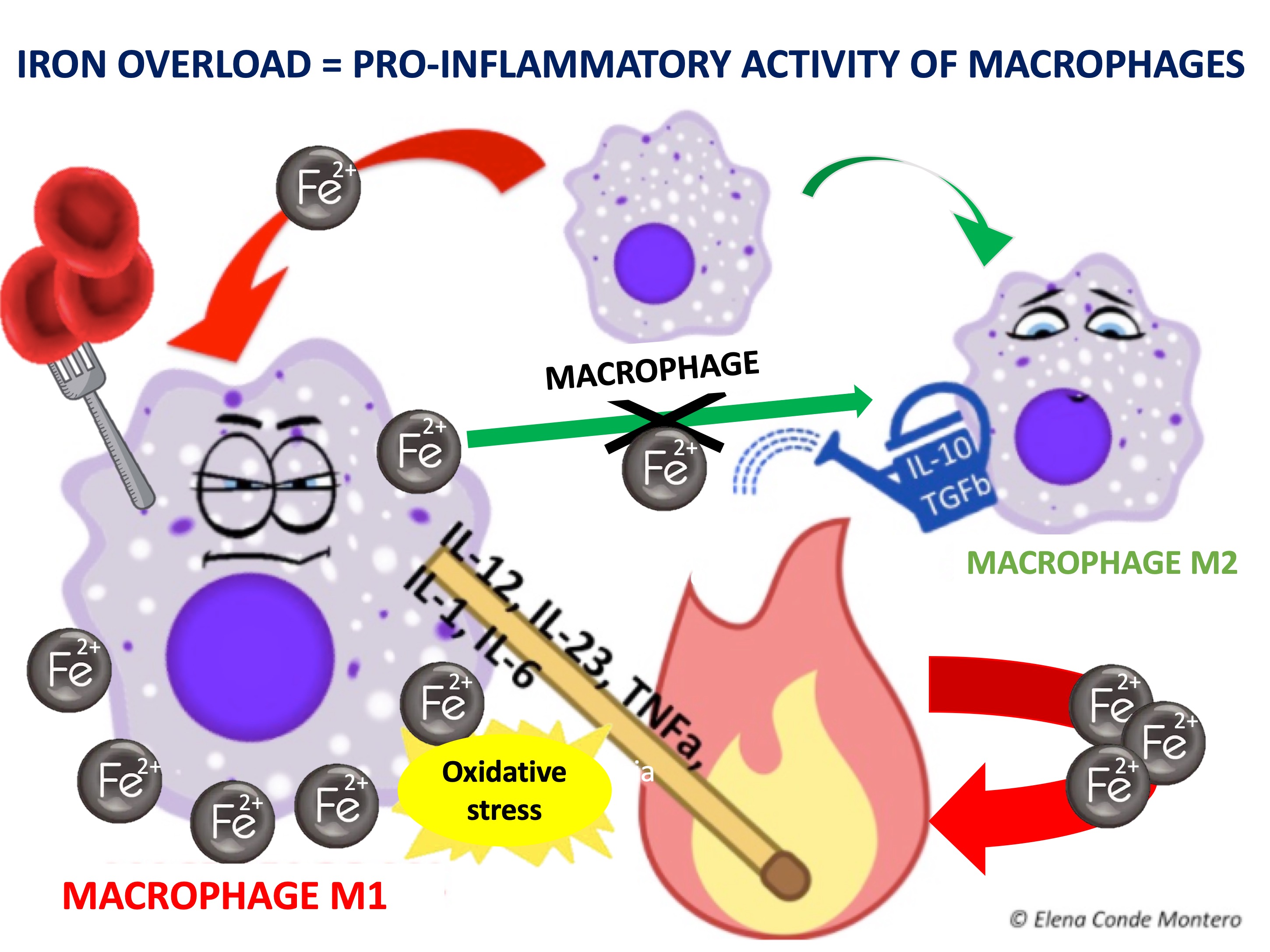
In other words, iron deposits favour the persistence of M1 macrophages and the consequent release of pro-inflammatory cytokines. In addition, free oxygen radicals released by the chemical reactions of iron ions in macrophages cause the accumulation of senescent fibroblasts resistant to apoptosis, which also release pro-inflammatory factors.5 This pro-inflammatory microenvironment produces more blood extravasation and, therefore, more iron deposits, producing a vicious circle.
What could be done to control the pro-inflammatory action of iron deposits?
The most reasonable would be to look for a method of chelating (sequestering) iron within the wound to prevent the release of reactive oxygen species by the ionic iron. Deferoxamine is an established iron chelator as a systemic treatment for iron overload situations such as hemochromatosis.
Would a deferoxamine-impregnated dressing be useful? More than 15 years ago, specific in vitro studies were published that pointed to the interest of this chelator applied topically…6,7Hmmm Why has no further research been done in this line?
Hopefully further studies will be carried out to find out the real role of these iron deposits in the chronicity of venous ulcers and, therefore, their potential role as a therapeutic target!
Referencias:
- Ferris AE, Harding KG. Does localized iron loss in venous disease lead to systemic iron deficiency? A descriptive pilot study. Wound Repair Regen. 2020 Jan;28(1):33-38.
- Ferris AE, Harding KG. An overview of the relationship between anaemia, iron, and venous leg ulcers. Int Wound J. 2019 Dec;16(6):1323-1329.
- Caggiati A, Rosi C, Casini A, et al. Skin iron deposition characterises lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg. 2010;40(6):777-782.
- Caggiati A, Rosi C, Franceschini M, Innocenzi D. The nature of skin pigmentations in chronic venous insufficiency: a preliminary report. Eur J Vasc Endovasc Surg. 2008;35(1):111-118.
- Wlaschek M, Singh K, Sindrilaru A, Crisan D, Scharffetter-Kochanek K. Iron and iron-dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non-healing chronic wounds. Free Radic Biol Med. 2019 Mar;133:262-275.
- Wenk J, Foitzik A, Achterberg V, et al. Selective pick-up of increased iron by deferoxamine-coupled cellulose abrogates the iron-driven induction of matrix-degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J Invest Dermatol. 2001;116(6):833-839.
- Taylor JE, Laity PR, Hicks J, et al. Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds. Biomaterials. 2005;26(30):6024-6033.
Also available in: Español (Spanish)